There’s been a lot of talk about testing folk for COVID-19. It would seem blindingly obvious testing is important. It’s not just about people knowing their status so they can get the right care and reduce the possibility of their infecting others, it’s also crucial to inform an evidence-based response to the pandemic by the authorities.
It also allows critical workers like those in healthcare to know if it’s safe to go back to work.
But as ever with biology, it’s complicated. I don’t want to get into the politics of mass testing; for this post I just want to do the nerdy bit about how the different tests actually work. It’s wankingly cool so put the kettle on, pull up a chair and let’s do some biochemistry porn…
How?
There’s a number of methods available to diagnose COVID-19 but each has its own strengths and weaknesses.
Genetic: PCR
This stands for Polymerase Chain Reaction. This is where you get a nose or throat swab and see if you can find the viral genetic material (RNA) in it. The PCR bit amplifies the signal by (usually) converting the RNA into DNA and then copying it many times until there’s enough to actually do the test.
Without getting into the fine detail of all the different PCR techniques now available it’s the same process used to amplify DNA recovered from crime scenes – and its inventor, Kary Mullis, got a Nobel for it.
But even though PCR exhibits both sensitivity and specificity it has its drawbacks; in the early stage of the disease the viral load might be too low to be detected or the subject might not have have major respiratory symptoms – and so little detectable virus in the nose and throat. You also need really good technique; it’s a very sensitive assay and samples are easily contaminated.
So, in many respects PCR is the ‘gold standard’ but it’s also time consuming, finicky and expensive.
Serology
This is the test that’s being rolled out now. It involves looking for evidence of the body’s reaction to the virus found in blood serum (or lymph or other extracellular fluid) – and it just needs a finger-prick of blood, something that looks like a pregnancy piss-stick and about 10 minutes. No labs. No mucking about.
Here’s how it works.
When our bodies are challenged by an invader, the body can mount both specific and non-specific defences. I want to concentrate on one specific response for now, antibodies.
An antibody or immunoglobulin (Ig) is a large protein used by the immune system to neutralise pathogens like bacteria and viruses. Individual antibodies are specific to a particular challenge and there are tons of them, one for each specific antigen you’ve ever been exposed to.
Antibodies recognise a molecule unique to each different pathogen (an antigen) and lock on to it. It’s like they recognise the fingerprint of a known offender. By attaching it tags a microbe or an infected cell for attack by other parts of the immune system. In some cases antibodies can neutralise the target directly by binding to a part of it that it needs to do its stuff and thus blocking it.
One of the first antibodies to be produced in an infection is IgM. It appears early in the course of an infection, then disappears but can reappear after subsequent exposure to the antibody if the person is reinfected.
The other class of antibody of interest here is IgG. It’s the most common type of antibody found in the body and does lots of cool stuff – but what’s of interest here is it stays in the blood and extracellular fluids after the infection has passed and after both IgM and any viral RNA have long gone.
You can see what I mean here:
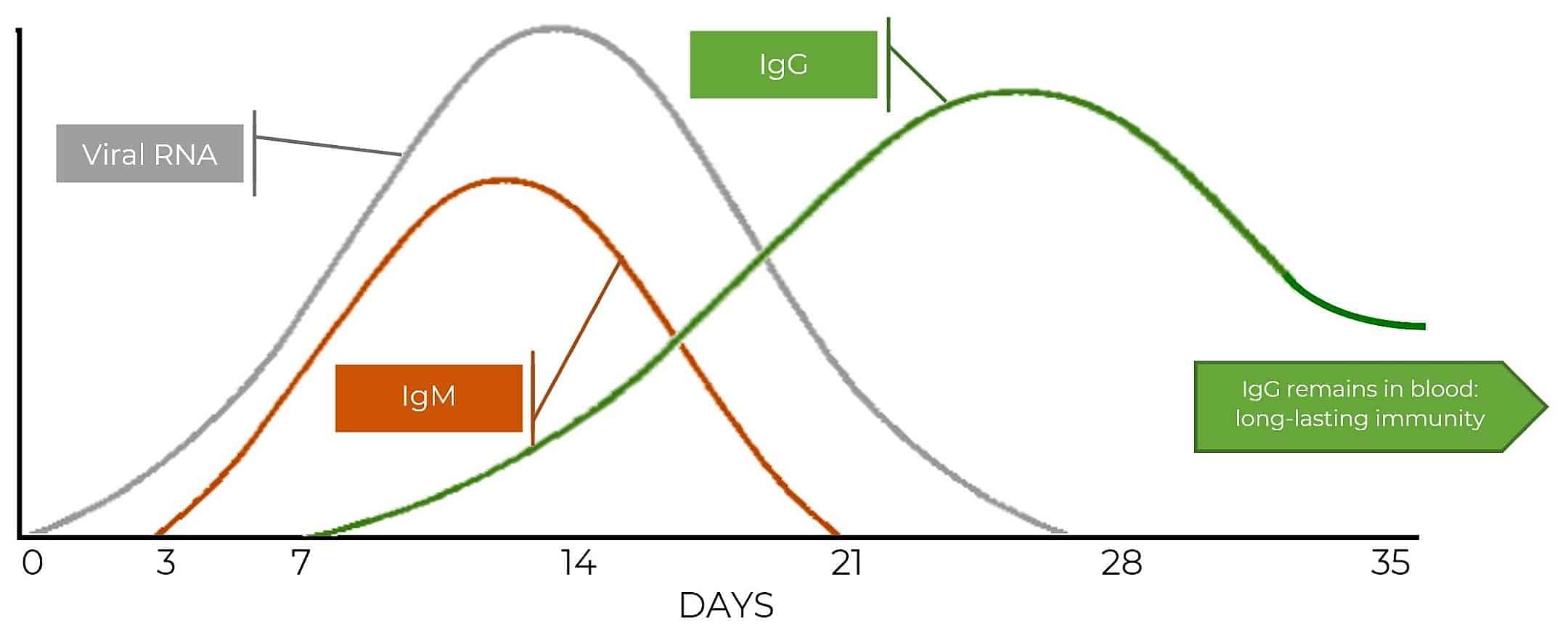
So, viral RNA (from virus particles) is there from Day 1 of infection and if you can find it there’s a very specific test for it. Three days in IgM turns up and (in the case of COVID-19) is detectable until the end if the third week.
IgG doesn’t arrive until the end of Week 1 – but after that it’s around for good. It’s only in small – but detectable – quantities: it’s one of the parts of the immune system that’s keeping a watching brief for future infection. If the IgG comes across the specific antigen it was raised against it alerts the other wings of the immune system and starts a new response – and rapidly.
The Test
So, a couple of days or so after onset of symptoms the presence of antibodies tells us whether someone has been exposed to the virus and gives us a steer as to their likely status.
The test itself looks like a pregnancy test piss-stick sort of affair. There are several on the market but all work in the same way. Because blood is thicker than pee there’s a well to put a pinprick of blood into and another for a buffer solution – the buffer carries the blood plasma into the bit of the cassette where the clever stuff happens. Remember doing chromatography at school? Just like that.
The sample of blood goes in the well (S), the buffer is added to (B). A few minutes later a number of things might happen:
Assuming you do PCR and serology, here’s the permutations:
| PCR | IgM | IgG | Interpretation |
|---|---|---|---|
| + | – | – | Early infection – window period |
| + | + | – | Early stage of infection |
| + | + | + | Active phase of infection |
| + | – | + | Late or recurrent infection |
| – | + | – | Probably early stage and PCR false-negative |
| – | – | + | Past infection and has recovered |
| – | + | + | Recovery stage and PCR false-negative |
There is some other stuff you need to do when designing these like ensuring nothing else in the sample – blood in this case – interferes with the result.
So, antibody tests are quick to perform (10mins max), unlike PCR they don’t need any special kit or trained technicians and are pretty accurate (87% for IgM, 97% for IgG).
Even better, they can rapidly give an indication of what stage in any infectious process an individual is and can tell if someone has immunity long after the virus has cleared. What’s not to like?



Why did we get caught with no kits ?
Tony Taylor Because it’s just been/being developed, as the article says.
Tony Taylor I should add, as I know a little bit about this having done a bit of work related to drug immunoassays back in the dawn of time, that the antibodies are specific to a virus, and of course the virus itself was unknown until the end of last year. So the assay has to be very specific – there’s loads of different antibodies in our blood, and it has to ignore them or it would give a false positive. I don’t want to claim knowledge I don’t have, but it seems pretty quickly developed to me.
Tony – PCR of various flavours has been around since January. How much it’s been used in UK is a different question.
Immunoassay was only licensed last week, I think. Reports on the point of care tests I’ve seen were dated 20th.
Sorry I was being naïve – why have the goons not been told of this reality ?
I think much of the reporting (and pretty much everything on FaceAche) centres around making cheap political points and not facts.
Sean Derrig listening to our new stand in PM earlier the press seem to be trying hat all the time !!!!